Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide.
CaCO3(s) 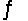
CaO(s)+ CO2(g)
KP for this reaction is 1.16 at 800°C.A 5.00 L vessel containing 10.0 g of CaCO3(s)was evacuated to remove the air, sealed, and then heated to 800°C.Ignoring the volume occupied by the solid, what will be the overall mass percent of carbon in the solid once equilibrium is reached?
Definitions:
Signoria
A form of government in Italian city-states during the medieval and Renaissance periods, characterized by rule by a single lord or a hereditary ruling family.
Medici
A powerful and influential family in Florence, Italy, during the Renaissance, known for their support of the arts, significant political control, and the production of four popes.
Merchant Capitalism
An early phase in the development of capitalism where trade and the merchant class played a primary role in economic life.
Condottieri
Condottieri were leaders of mercenary soldiers in Italy during the Middle Ages and Renaissance, hired by city-states and the papacy for military purposes.
Q6: Consider the following equilibrium,<br>4NH<sub>3</sub>(g)+ 3O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q18: For the reaction HCONH<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q19: Which one of these equations represents
Q35: Each of the following substances is a
Q44: Which one of the following reagents is
Q63: Indicate all the types of intermolecular forces
Q90: For the reaction H<sub>2</sub>(g)+ S(s) <span
Q94: For H<sub>3</sub>PO<sub>4</sub>, K<sub>a1</sub> = 7.3 * 10<sup>-3</sup>,
Q97: Identify the dominant (strongest)type of intermolecular force
Q108: The solubility of a salt increases as