Predict the direction in which the equilibrium will lie for the reaction H2CO3 + F-
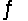 HCO3- + HF. Ka1( H2CO3) = 4.2 * 10-7; Ka(HF) = 7.1 * 10-4
HCO3- + HF. Ka1( H2CO3) = 4.2 * 10-7; Ka(HF) = 7.1 * 10-4
Definitions:
Granulosa Cells
Cells that surround developing eggs in the ovary and secrete hormones important for egg maturation and the menstrual cycle.
Telolecithal Eggs
Eggs containing a large amount of yolk concentrated at one pole, common in birds, reptiles, and some fish.
Notochord
A flexible rod-like structure that forms the main support of the body in all embryonic and some adult chordate animals.
Neural Plate
A key embryonic structure that develops into the nervous system, including the brain and spinal cord.
Q12: The reaction 2SO<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="The reaction
Q18: Appropriate units for a second-order rate constant
Q28: The rate constant of the reaction
Q32: Which of the following liquids would make
Q47: Determine the equilibrium constant, K<sub>eq</sub>, at
Q49: For the chemical reaction system described by
Q60: How does the entropy change when a
Q87: What is the pH of an aqueous
Q91: Chlorine dioxide reacts in basic water to
Q125: The molar enthalpy of vaporization of boron