Predict the direction in which the equilibrium will lie for the reaction H2SO3(aq) + HCO3- (aq) 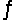 HSO3-(aq) + H2CO3(aq) .
HSO3-(aq) + H2CO3(aq) .
Ka1(H2SO3) = 1 * 10-2; Ka1(H2CO3) = 4.2 * 10-7
Definitions:
Fixed Cash Receipts
Fixed Cash Receipts refer to the regular, unchanging amount of cash received by a business or individual, typically structured within certain financial arrangements or revenue models.
Discount Rate
The interest rate used in discounted cash flow analysis to determine the present value of future cash flows.
Lag Strategy
A deliberate decision to not be a first mover in an industry or market, observing and reacting to competitors' actions.
Straddle Strategy
A trading strategy that involves purchasing both a call option and a put option for the same underlying asset, with the same strike price and expiration date, allowing investors to benefit from significant price movements in either direction.
Q26: How many grams of copper are deposited
Q27: Calculate the amount of heat that
Q36: For the reaction PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q51: Calculate E°<sub>cell</sub> for a silver-aluminum cell
Q64: At 35ºC, the equilibrium constant for the
Q76: Consider the following equilibrium,<br>4NH<sub>3</sub>(g)+ 3O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q91: Bromothymol blue is a common acid-base indicator.
Q95: For the reaction SbCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q112: Consider the following standard reduction potentials in
Q118: How many coulombs of charge are