Multiple Choice
Predict the direction in which the equilibrium will lie for the reaction H3PO4 + NO3- 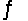 H2PO4- + HNO3 Ka(H3PO4) = 7.5 * 10-3
H2PO4- + HNO3 Ka(H3PO4) = 7.5 * 10-3
Definitions:
Related Questions
Q1: Write out the steps that show how
Q12: The equilibrium constant for the reaction C<sub>7</sub>H<sub>15</sub>COOH(aq)+
Q28: When the reaction 2H<sub>2</sub>S(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="When
Q29: What is the concentration of O<sub>2</sub>(g)in water
Q39: A solution is prepared by adding 40.3
Q53: Calculate the H<sup>+</sup> ion concentration in a
Q57: Using a table of standard electrode potentials,
Q60: How does the entropy change when a
Q76: Ethanol and dimethyl ether have the same
Q90: For the reaction H<sub>2</sub>(g)+ S(s) <span