In the gas phase, methyl isocyanate (CH3NC) isomerizes to acetonitrile (CH3CN) , H3C-N C (g) 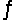 H3C-C N (g)
H3C-C N (g)
With H° = -89.5 kJ/mol and G° = - 73.8 kJ/mol at 25°C. Find the equilibrium constant for this reaction at 100°C.
Definitions:
Allocating Cost
The process of assigning costs to various cost objects such as products, services, or departments for accounting purposes.
Inventory Accounting
A method of accounting that deals with valuing and accounting for changes in inventoried assets, impacting how businesses report their cost of goods sold and value of inventory in financial statements.
Just-In-Time Systems
Inventory management systems that produce or provide items exactly when needed, minimizing inventory costs.
Stock-Outs
Stock-outs occur when an item is no longer available for sale, typically because inventory levels have been depleted.
Q2: If the reaction 2H<sub>2</sub>S(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="If
Q10: The Ostwald process is the main method
Q14: Bidentate and polydentate ligands are also called
Q25: The <sup>14</sup>C activity of some ancient Peruvian
Q42: In comparing three solutions with pH's of
Q47: The electron configuration of a Ti atom
Q73: The rate law predicted by the following
Q77: What is the nuclear binding energy
Q85: Which response gives the products of hydrolysis
Q110: What current is needed to deposit 0.500