Find the temperature at which the reaction N2O4(g) 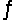 2NO2(g) will be in equilibrium when both gases are present at partial pressures of 1.00 atm.
2NO2(g) will be in equilibrium when both gases are present at partial pressures of 1.00 atm. 
Definitions:
Leader Reward Behaviour
A leadership approach that involves the use of positive reinforcement to encourage desirable behaviors in followers.
Role Ambiguity
The uncertainty and lack of clarity about what one's role is within an organization, including responsibilities, expectations, and boundaries.
Positive Perceptions
The tendency to view situations, actions, or conditions in a favorable light.
Contingency Variable
A variable that modifies the relationship between two other variables under certain conditions or situations.
Q1: For the reaction CuS(s)+ H<sub>2</sub>(g) <img
Q20: Complete and balance the following redox
Q24: For the reaction CuS(s)+ H<sub>2</sub>(g) <img
Q31: A 0.10 M NH<sub>3</sub> solution is 1.3%
Q46: Which two compounds are produced by the
Q56: The oxidation of iodide ions by arsenic
Q62: Calculate the cell emf for the
Q86: The following reactions occur at 500
Q90: The mass of a nucleus is always
Q110: Charcoal samples taken from holes dug at