For the reaction CuS(s)+ H2(g) 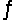 H2S(g)+ Cu(s),
H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= - 20.6 kJ/mol
Will this reaction proceed spontaneously at 298 K and 1 atm pressure?
Definitions:
Departmental Contributions
The financial or resource contributions made by individual departments towards the achievement of the overall organizational goals.
Direct Expense
Direct expense refers to costs directly incurred in the manufacturing of a product or in providing a service, distinguishable by its direct association with specific business activities.
Indirect Expenses
Costs that are not directly attributable to the production of goods or services, such as administrative and marketing expenses.
Investment Center
A business unit within a company that is responsible for its own revenue, expenses, and investment of capital, often evaluated on its return on investment.
Q6: Consider the following equilibrium,<br>4NH<sub>3</sub>(g)+ 3O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q14: Ozone (O<sub>3</sub>)and oxygen gas (O<sub>2</sub>)are examples of<br>A)isotopes.<br>B)allotropes.<br>C)polymorphs.<br>D)alloys.<br>E)amphoterism.
Q17: If 12% of a certain radioisotope decays
Q28: The rate constant of the reaction
Q30: Consider the reaction Fe + Sn<sup>2+</sup>(1
Q36: For the reaction PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q37: A rock contains 0.37 mg of
Q97: Rain collected on a remote island in
Q101: Calculate the pH of a 6.71 *
Q157: Morphine, C<sub>17</sub>H<sub>19</sub>NO<sub>3</sub>, is often used to control