Multiple Choice
Determine the equilibrium constant (Keq) at 25°C for the reaction Cl2(g) + 2Br- (aq) 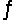
2Cl- (aq) + Br2(l)
Definitions:
Product Decision
The selection, definition, and design of products.
Related Questions
Q1: How many structural isomers are there of
Q24: Will a precipitate of magnesium fluoride form
Q28: Which one of these molecules could not
Q34: Nitrosyl chloride (NOCl)decomposes at elevated temperatures
Q38: What is the pH of 10.0 mL
Q54: Write the chemical formula of magnetite.
Q88: What is the pH at the equivalence
Q93: Suppose that ammonia, applied to a field
Q102: Given the following standard reduction potentials,
Q123: An aqueous solution of KCl would be<br>A)neutral<br>B)basic<br>C)acidic