Determine the equilibrium constant for the following reaction at 298 K.
2Fe3+(aq)+ H2O2(aq) 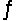 2H+(aq)+ O2(g)+ 2Fe2+(aq).
2H+(aq)+ O2(g)+ 2Fe2+(aq).
Definitions:
Income Statement
A record detailing a firm's income, costs, and gains during a particular timeframe.
Depreciation Expense
The allocation of the cost of a tangible asset over its useful life, reflecting the asset's wear and tear, decay, or decline in value.
Accumulated Depreciation
The total amount of depreciation expense that has been recorded against a fixed asset since it was acquired and put into use.
Income Statement
A financial statement that shows a company's revenues, expenses, and profits over a specific period.
Q6: For the reaction SbCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q13: In the reaction HNO<sub>3</sub> + NH<sub>3</sub> <img
Q34: The only true hydrocarbon polymer found in
Q34: Cast iron as it is prepared in
Q50: Consider the reaction Pb(s)+ 2H<sup>+</sup>(aq) <span
Q71: The neutral monodentate ligand L forms the
Q75: Stay Dry Raincoats uses three different machines
Q87: What volume of 0.0500 M sodium hydroxide
Q90: If the pH of an acid rain
Q101: Consider the gas phase reaction shown