The equilibrium 3Fe(s) + C(s) 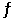 Fe3C(s) is established in a solid solution. For such a solution, one can write an equilibrium constant in the usual way except that here one has concentrations that refer to solids in the solid solution. Determine the equilibrium constant for the formation of cementite from iron and carbon at 680°C.(Given: for this reaction at 25°C, H° = 21 kJ/mol and S° = 20.4 J/mol·K)
Fe3C(s) is established in a solid solution. For such a solution, one can write an equilibrium constant in the usual way except that here one has concentrations that refer to solids in the solid solution. Determine the equilibrium constant for the formation of cementite from iron and carbon at 680°C.(Given: for this reaction at 25°C, H° = 21 kJ/mol and S° = 20.4 J/mol·K)
Definitions:
Cost Principle
An accounting principle that states assets should be recorded at their cost at the time of purchase, not their current value.
Q2: Ozone is formed in the atmosphere by
Q5: Predict the sign of <span
Q6: For the reaction SbCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q13: In which one of the following solutions
Q40: Find the emf of the cell described
Q44: Which response gives the correct coordination number
Q45: Beryllium, the first element in Group 2A,
Q45: Given the following data, calculate the boiling
Q55: Balance the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="Balance the
Q124: The equilibrium constant for the reaction C<sub>6</sub>H<sub>5</sub>COOH(aq)+