Multiple Choice
At 1000.0 K, the equilibrium constant for the reaction 2NO (g) + Br2 (g) 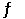 2NOBr (g)
2NOBr (g)
Is Kp = 0.017. Calculate Kp for the reverse reaction,
2NOBr (g) 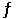 2NO (g) + Br2 (g) .
2NO (g) + Br2 (g) .
Definitions:
Related Questions
Q13: The layer of the atmosphere that contains
Q19: The standard Gibbs free energy of formation
Q20: What two metals are alloyed to produce
Q32: The equilibrium constant for the gas phase
Q49: The solubility of MnSO<sub>4</sub> monohydrate in water
Q59: On the phase diagram shown above, the
Q60: The value of ΔH° for the formation
Q70: The amount of atomic O relative to
Q85: The combustion of ethylene proceeds by the
Q95: What is the molality of sodium chloride