Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1) .The initial pressure,number of moles,and temperature of the gas are noted on the diagram.Which diagram (2) -(4) most closely represents the result of doubling the temperature while keeping the pressure and number of moles of gas constant? 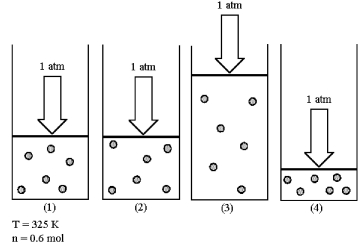
Definitions:
Downward Move
Assignment of an employee to a position with less responsibility and authority.
In-basket Exercises
A simulation exercise used in assessment centers, mimicking the workload and decision-making challenges faced in a job.
Documents
Written, printed, or electronic records that provide information or evidence.
Q23: Which of the following contains an atom
Q35: What is the geometry around the central
Q35: How much heat is absorbed/released when 20.00
Q90: Calculate the total quantity of heat required
Q94: What is the pressure (in mm Hg)of
Q97: A solution is prepared by dissolving 17.75
Q111: For the hypothetical second order reaction: A
Q119: When 12.0 g of zinc metal reacts
Q128: From the phase diagram above,the minimum pressure
Q183: Each of three identical 15.0-L gas cylinders