Consider a reaction that occurs by the following one-step mechanism:
A2 + B2 → 2 AB
The potential energy profile for this reaction is shown below. 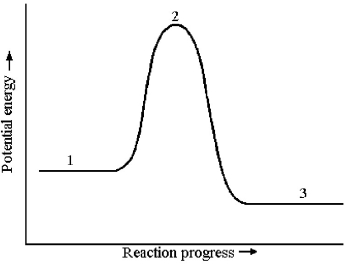
-What is the species present at reaction stage 3?
Definitions:
Corporations
Entities established to conduct business, characterized by having legal rights, responsibilities, and a structure that separates them from their members.
Human Identity
encompasses the traits, experiences, relationships, and values that define a person as an individual, contributing to their sense of self and distinctiveness from others.
Active Participant
An individual who takes an engaged and contributory role in processes or activities, rather than being passive.
Dialogue
A conversation between two or more people as a feature of a book, play, or movie, or the process of engaging in conversation to resolve a dispute.
Q5: The activation energy for the forward reaction
Q29: The coordination number of each atom in
Q54: The following picture represents the equilibrium state
Q95: Which cation in each set would be
Q121: The reaction that occurs in a Breathalyzer,a
Q153: The pH of 0.150 M CH<sub>3</sub>CO<sub>2</sub>H,acetic acid,is
Q162: At a given temperature the vapor pressures
Q166: What is the strongest acid among the
Q191: Assuming that sea water is a 3.5
Q192: Identify the Br∅nsted-Lowry acid/base conjugate pairs.<br>A)(1)/(2)and (3)/(4)<br>B)(1)/(3)and