Multiple Choice
The following pictures represent solutions that contain a weak acid HA (pKa = 5.0) and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 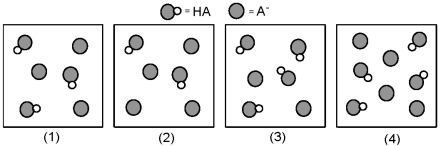
-For which of these solutions is pH = pKa?
Definitions:
Related Questions
Q1: Calculate the pH of a 0.020 M
Q13: For a galvanic cell that uses the
Q47: What is the hydronium ion concentration of
Q98: Addition of 0.0125 mol KOH to 150
Q100: What is the approximate pH of a
Q123: What is the quantitative change in the
Q135: What is K if ΔG° = -19.0
Q145: Given the hypothetical reaction: 2 A(s)+ x
Q195: A solution with a hydroxide ion concentration
Q198: Which picture represents the system halfway between