Multiple Choice
What is the hydronium ion concentration in a solution prepared by mixing 50.00 mL of 0.10 M HCN with 50.00 mL of 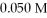 NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
Definitions:
Related Questions
Q7: What is the species present at reaction
Q8: What are the Br∅nsted-Lowry acids in the
Q9: Which of the following changes in reaction
Q39: Write the equilibrium equation for the forward
Q41: Identify the Br∅nsted-Lowry acid/base conjugate pairs.<br>A)(1)/(2)and (3)/(4)<br>B)(1)/(3)and
Q93: At 298 K,K<sub>p</sub> = 2.1 × 10<sup>4</sup>
Q107: What is the shorthand notation that represents
Q114: Calculate the pH for an aqueous solution
Q122: Vinegar is a 5.0% solution by weight
Q153: At 25°C,a certain first order reaction has