Using the table of bond dissociation energies,the ΔH for the following gas-phase reaction is ________ kJ. 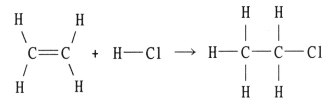
Definitions:
Plato
An ancient Greek philosopher, student of Socrates, and teacher of Aristotle, known for his contributions to philosophy, mathematics, and the concept of forms.
Saint Augustine
An early Christian theologian and philosopher whose writings influenced the development of Western Christianity and Western philosophy, especially regarding concepts of original sin and divine grace.
Aristotle
An ancient Greek philosopher and scientist, whose works cover a wide range of subjects and form the basis of Western philosophy.
Aristotle
An ancient Greek philosopher and scientist, one of the greatest intellectual figures of Western history, who made significant contributions to various fields of knowledge.
Q4: A closed-end manometer was attached to a
Q20: Electromagnetic radiation with a wavelength of 641
Q40: A positive change in bond enthalpy is
Q47: How much heat is required to raise
Q75: Of the following metals,_ exhibits multiple oxidation
Q81: Electron domains for single bonds exert greater
Q83: Using the table of bond dissociation energies,the
Q94: What is the maximum number of double
Q119: Based on molecular orbital theory,the bond order
Q139: Using the VSEPR model,the molecular geometry of