2.0 L of a ideal nitrogen gas (N2)are at 0.00°C and 1.0 atm.The ideal gas constant is 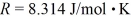 = 0.0821 L • atm/mol • K,Avogadro's number is 6.022 × 1023 molecules/mol,and the ATOMIC mass of nitrogen is 14 g/mol.
= 0.0821 L • atm/mol • K,Avogadro's number is 6.022 × 1023 molecules/mol,and the ATOMIC mass of nitrogen is 14 g/mol.
(a)Determine the number of moles of N2.
(b)How many molecules of N2 are present?
(c)What is the mass of this gas?
Definitions:
Weighted Average Model
A calculation that assigns different weights to different items or data points according to their importance in achieving a more accurate average.
Forecasts
Predictions or estimates of future events or trends, based on analysis of available data and indicators.
Mean Absolute Deviation
A statistical metric that measures the average distance between each data point and the mean of a data set, demonstrating variability.
Weighted Average Model
A calculation model that assigns varying degrees of importance or weights to different data points or variables when computing an average.
Q5: A simple harmonic oscillator has an amplitude
Q6: A sphere is constructed of two concentric
Q7: Three capacitors are arranged as shown in
Q16: In a thermodynamic process involving 7.8 moles
Q16: A 0.25 kg ideal harmonic oscillator has
Q24: A 2.00-m long piano wire with a
Q28: The number of molecules in one mole
Q31: Two planets having equal masses are in
Q33: Four dipoles,each consisting of a +10-µC charge
Q35: A uniform solid cylinder of radius R