The following reaction occurred when a 1.0-liter reaction vessel was initially charged with 2.0 moles of N2(g) and 4.0 moles of H2(g) :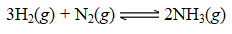
Once equilibrium was established,the concentration of NH3(g) was determined to be 0.59 M at 700.°C.The value for Kc at 700.°C for the formation of ammonia is:
Definitions:
Neuropsychology
The study of the relationship between brain function and behavior, including the effects of brain injuries and diseases.
Trait Theory
A psychological approach focused on the quantification and analysis of personality traits or characteristics that are consistent over time and situation.
Positive Reinforcement
Involves the addition of a rewarding stimulus following a desired behavior, increasing the likelihood of that behavior being repeated.
Negative Punishment
A behavior modification technique where a desirable stimulus is removed after a particular behavior, with the aim of decreasing that behavior.
Q5: Dinitrogen monoxide,or laughing gas,can be made
Q13: What is the pH of a
Q17: Given the following equilibria,<br>PbBr<sub>2</sub>(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q21: Calculate the equilibrium constant for the
Q36: Does the formation of complex molecules such
Q58: Which of the following mathematical expressions is
Q60: In the United States,most sulfur is obtained
Q68: Which of the following compounds has the
Q76: Which one of the following elements would
Q87: What is the pH of the