Assume that the following chemical reaction is at equilibrium.
C(s) + H2O(g) 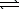 H2(g) + CO(g)
H2(g) + CO(g)
Which of the following statements is/are CORRECT?
1) Increasing the concentration of H2(g) will cause the reaction to proceed in the backward direction,increasing the equilibrium concentration of H2O(g) .
2) Decreasing the temperature will cause the reaction to proceed in the forward direction,increasing the equilibrium concentration of CO(g) .
3) Increasing the amount of C(s) will cause the reaction to proceed in the forward direction,increasing the equilibrium concentration of CO(g) .
Definitions:
Doubts
Feelings of uncertainty or lack of conviction about something, often involving questions about belief or decision making.
D-motivation
The reduction or loss of motivation, often due to a lack of rewards or the presence of demotivating factors.
Self-actualizers
Individuals who have realized and fulfilled their potential in terms of personal growth, creativity, and understanding, according to Maslow's hierarchy of needs.
Maslow
Refers to Abraham Maslow, a psychologist known for creating Maslow's hierarchy of needs, a theory in psychology that argues human behaviors are motivated by fulfilling a hierarchy of needs from basic to complex.
Q1: The following reaction occurred when a
Q6: Hyperventilation can cause your blood pH to
Q7: Which of the following liquids has
Q7: For a chemical reaction,if <span
Q9: Which of the following statements is/are CORRECT?<br>1)A
Q17: Which of the following are standard conditions
Q28: What is the mole fraction of urea,CH<sub>4</sub>N<sub>2</sub>O,in
Q34: Consider the following half-reactions:<br>Ag<sup>+</sup>(aq)+ e<sup>-</sup>
Q52: Which of the following statements concerning solubility
Q59: Which of the following reactions would