What is the equilibrium constant for the following reaction,
HCO2H(aq) + CN-(aq) 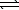 HCO2-(aq) + HCN(aq)
HCO2-(aq) + HCN(aq)
And does the reaction favor the formation of reactants or products? The acid dissociation constant,Ka,for HCO2H is 1.8 10-4 and the acid dissociation constant for HCN is 4.0 10-10.
Definitions:
Mailbox Rule
A legal principle stating that an offer is considered accepted once the acceptance is dispatched, not when it is received.
Effective Upon Receipt
A condition indicating that a document, notification, or agreement becomes legally binding as soon as it is received by the intended party.
Online Businesses
Online Businesses operate on the internet, selling goods or services directly to consumers through digital platforms without a physical storefront.
Full Disclosure
Obligation to provide all relevant information and facts to the other party, particularly in financial transactions or legal agreements.
Q11: What is the equilibrium pH of an
Q12: A solution containing 10.mmol of CO<sub>3</sub><sup>2-</sup> and
Q19: What is the value of the
Q29: Write a balanced chemical equation for
Q43: What is the mass of H<sub>2</sub>SO<sub>4</sub> in
Q44: A 50.00-mL solution of 0.0797 M
Q55: Assume that the following <span
Q55: Which of the following statements is true
Q65: A 12.0% sucrose solution by mass has
Q69: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span