An impure sample of sodium carbonate,Na2CO3,is titrated with 0.113 M HCl according to the reaction below.
2 HCl(aq) + Na2CO3(aq) 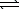 CO2(g) + H2O(
CO2(g) + H2O( 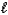 ) + 2 NaCl(aq)
) + 2 NaCl(aq)
What is the percent of Na2CO3 in a 0.613 g sample if the titration requires 26.14 mL of HCl? The molar mass of Na2CO3 is 106.0 g/mol.
Definitions:
Return
The gain or loss on an investment over a specified period, expressed as a percentage of the investment’s initial cost.
Residual Income
The amount of income that an entity generates after accounting for the cost of capital, indicating profitability beyond the minimum required return.
Investment Opportunity
A potential financial venture or asset that could generate income or appreciate in value over time.
Combined
Refers to the amalgamation or integration of two or more elements into a single unit or system.
Q1: The following reaction occurred when a
Q12: Calculate the pH of the following aqueous
Q17: Most hydrogen gas is produced industrially by<br>A)
Q17: In what type of unit cell are
Q25: At a given temperature,K = 0.021 for
Q42: Assume that the following chemical reaction
Q45: Elemental sodium is commercially produced by electrolysis
Q60: Which of the following equations shows that
Q69: What is the name of the following
Q69: If 0.50 L of a buffer containing