Which of the following reactions would be expected to have a positive entropy change, ∆rS° > 0?
1. 2 SO2(g) + O2(g) → 2 SO3(g)
2. Ba(OH) 2(s) → BaO(s) + H2O(g)
3. CO(g) + 2 H2(g) CH3OH( 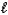 )
)
Definitions:
Pharmaceutical Companies
Companies engaged in the research, development, production, and marketing of drugs or medications.
Prescription Drug
Medication that legally requires a medical doctor's prescription to be dispensed.
Personal Selling
refers to a direct form of marketing where sales representatives personally meet with potential customers to pitch a product or service.
Free Samples
A marketing strategy where small quantities of the product are given away for free to consumers to try and increase awareness or sales.
Q1: Calculate the molar concentration of uncomplexed Zn<sup>2+</sup>(aq)in
Q1: In the following electrochemical cell,what is
Q5: When a Lewis acid and a Lewis
Q22: Which of the following occurs uncombined in
Q28: How many RNA nucleotides comprise a codon?<br>A)
Q41: How many electrons are transferred in the
Q44: A metal crystallizes in a face-centered cubic
Q59: Which of the following reactions would
Q68: When the following oxidation-reduction reaction in
Q77: What is the mole fraction of urea,CO(NH<sub>2</sub>)<sub>2</sub>,<sub>