Calculate S° for the reaction
SiCl4(g) + 2Mg(s) 2MgCl2(s) + Si(s) 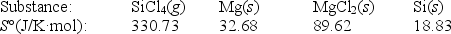
Definitions:
Widgets
A generic term often used to refer to a fictional or unspecified product or gadget.
Nonunion Firms
Companies or organizations where the workforce is not represented by a labor union, often resulting in different working conditions compared to unionized settings.
Foreign Producers
Companies or individuals located outside a given country that produce goods or services for that country's market.
Union Wages
Wages negotiated by labor unions on behalf of their members, often resulting in higher pay and better work conditions compared to non-unionized workers.
Q12: a.Draw two different structures with the molecular
Q19: Identify the products of the following reaction.
Q20: The halogens are<br>A)strong oxidizing agents.<br>B)strong reducing agents.<br>C)strong
Q52: Predict the products for the following
Q56: At 450°C,tert-butyl alcohol decomposes into water and
Q61: Which of the following isotopes is most
Q65: Select the nuclide that completes the following
Q68: Bonds that sell at a discount have
Q73: In some spontaneous processes,the entropy of the
Q83: The Downs cell is used in the