Consider the figure which shows G° for a chemical process plotted against absolute temperature. 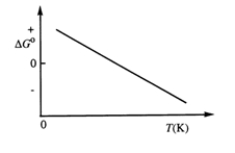 Which one of the following is an incorrect conclusion, based on the information in the diagram?
Which one of the following is an incorrect conclusion, based on the information in the diagram?
Definitions:
Marfan Syndrome
A genetic disorder that affects the body's connective tissue, leading to abnormalities in the heart, blood vessels, eyes, bones, and ligaments.
Achondroplastic Dwarfism
A genetic disorder characterized by short stature and disproportionately short limbs, the most common form of dwarfism.
Nutritional Status
An assessment of the level of nutrients in the body and the body’s ability to maintain and utilize them.
Body Stature
An individual's natural height and body build, often assessed in health and nutritional studies.
Q17: Increasing the initial amount of the limiting
Q30: A CH<sub>3</sub>COOH/CH<sub>3</sub>COO<sup>-</sup> buffer can be produced by
Q33: The elementary reaction HBr(g) + Br(g) <font
Q35: Tetrahedral complexes can exhibit both optical and
Q42: In the expression, S = k ln
Q45: Palladium-107 undergoes <font face="symbol"></font> decay (t<sub>1/2</sub> =
Q45: The decomposition of SOCl<sub>2 </sub>is first-order in
Q50: At high temperatures, carbon reacts with O<sub>2</sub>
Q62: What is the pH of a 0.0035
Q63: The specific heat capacity c of a