Consider the reaction of iodine with manganese dioxide 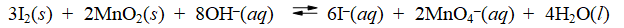
The equilibrium constant for the overall reaction is 8.30 × 10-7. Calculate G° for the reaction at 25°C.
Definitions:
Unanswered Questions
Queries or areas of inquiry that have not yet been addressed or resolved, often highlighting gaps in current knowledge or research.
Semantic Differential Scales
A type of survey rating scale used to measure the meaning of objects, events, or concepts, characteristically involving bipolar adjectives.
Basic Dimensions
Fundamental measurements or aspects that define the structure of a subject or concept, often used in physics and psychology.
Evaluation
The systematic assessment of the value, importance, or quality of something, often for the purpose of making informed decisions.
Q6: A noncoring needle is used to access
Q7: Implanted ports are used only for administration
Q9: The process of converting metal sulfides to
Q29: Alternating between venipuncture and dermal puncture collection
Q37: In tables of thermodynamic data provided in
Q42: In the expression, S = k ln
Q51: Aqueous solutions of phosphoric acid and sodium
Q61: Apply the valence bond theory to predict
Q74: Of the 3d transition series of elements,
Q84: What is the difference between a coordination