For the equilibrium 2 HI(g) H2(g) + I2(g) ,Kp = 29.1 at 1000.0 K.What is Kc at 1000.0 K for the equilibrium 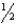 H2(g) +
H2(g) + 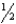 H2(g) HI(g) ?
H2(g) HI(g) ?
Definitions:
Average Annual Revenue
This refers to the amount of money a company earns in a year, on average, from its operations.
Standard Deviation
A metric indicating the extent to which data points diverge from the mean, showing the distribution's spread.
Average Annual Revenue
The mean income generated by a business, entity, or asset within one fiscal year.
Random Sample
A group of people selected from a bigger group, with every person having the same probability of being chosen.
Q6: Which of the following statements regarding the
Q10: Given the following data,calculate the approximate
Q28: Which of the following statements regarding coupled
Q36: Co(NH<sub>3</sub>)<sub>6</sub><sup>3+</sup>(aq)absorbs light in the blue region of
Q56: Glycolic acid,which is a monoprotic acid and
Q102: Using the thermodynamic data below,determine the
Q111: A Lewis acid is _<br>A)a proton donor.<br>B)a
Q113: Which of the following processes is NOT
Q117: The correct name for [Co(NH<sub>3</sub>)<sub>5</sub>Cl]Cl<sub>2</sub> is _<br>A)chloropentamminecobalt(III)chloride.<br>B)pentamminechlorocobalt(II)chloride.<br>C)pentamminechlorocobalt(III)chloride.<br>D)pentamminechlorocobaltate(III)chloride.<br>E)pentamminedichlorocobalt(III).
Q228: What would happen to the Ag<sup>+</sup> and