Consider the galvanic cell shown below (the contents of each half-cell are written beneath each compartment) : 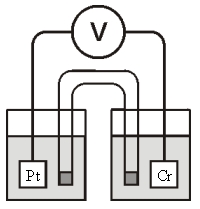 0.50 M Br2 0.20 M Cr3+ 0.10 M Br-
0.50 M Br2 0.20 M Cr3+ 0.10 M Br-
The standard reduction potentials are as follows:
Cr3+(aq) + 3e- Cr(s) =-0.727 V
Br2(aq) + 2e- 2Br-(aq) = +1.090 V
What is for this cell?
Definitions:
Bond Issues
The act of distributing new bonds to investors, typically done by corporations or governments to raise capital.
Glass-Steagall Act
US legislation enacted in 1933 to separate commercial banking from investment banking, aiming to reduce financial market risks.
Underwriting Securities
The process through which investment banks issue new securities to the market, assuming the risk of selling them at a certain price.
Accepting Deposits
A banking activity that involves receiving money from customers for safekeeping or for earning interest, typically in savings or checking accounts.
Q5: What is the pH at point III?<br>A)4.10<br>B)7.95<br>C)11.79<br>D)12.39<br>E)none
Q7: As water is heated,its pH decreases.This means
Q20: Given that <span class="ql-formula" data-value="\Delta"><span
Q24: Ethylenediamine (en)is a bidentate ligand.What is the
Q46: If an electrolysis plant operates its
Q56: Calculate the pH of the following aqueous
Q60: Copper is electroplated from CuSO<sub>4</sub> solution.A
Q67: Consider a certain type of nucleus that
Q111: When current is allowed to flow,which species
Q122: A monoprotic weak acid when dissolved