i. If the sales, production or other data over a period of time tend to approximate a straight-line trend, the equation developed by the least squares method cannot be used to forecast sales for a future period. ii. A straight-line trend equation is used to represent the time series when it is believed that the data is increasing(or decreasing) by equal amounts, on the average, from one period to another.
iii. If the past data approximates a straight line, the equation used is 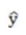 = a + bt, where a is they-intercept and b is the slope of the line.
= a + bt, where a is they-intercept and b is the slope of the line.
Definitions:
Ionic Compound
An ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding, typically formed between metal cations and non-metal anions.
Phosphorus
A reactive, non-metallic element found in Group 15 of the periodic table, essential for life.
Calcium
A chemical element with the symbol Ca and atomic number 20, a reactive, pale yellow metal that forms compounds with many other elements.
Ionic Formula
A notation that represents the composition of an ionic compound by showing the ratio of ions present in the compound.
Q6: IgM is the first antibody isotype secreted
Q20: B-1 B cells are considered a component
Q24: The variable lymphocyte receptors (VLRs) of lampreys
Q26: Real income is computed by:<br>A) dividing money
Q27: The immune system evolved to protect us
Q29: Angela Chou has been asked to investigate
Q34: You are trying to decide in which
Q40: i. The irregular component of a time
Q69: Teton Village contains shops, restaurants and motels.
Q94: A manager at a local bank analyzed