A scientist conducts an experiment to determine the rate of the following reaction: 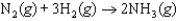 If the initial concentration of H2 was 0.150 M and the concentration of H2 was 0.050 M after 2.10 s, what is the average rate of the reaction?
If the initial concentration of H2 was 0.150 M and the concentration of H2 was 0.050 M after 2.10 s, what is the average rate of the reaction?
Definitions:
Pure Monopoly
A market structure where a single seller completely dominates the market for a product that has no close substitutes, allowing it to set prices without direct competition.
Profit-maximizing
A strategy or approach aimed at identifying the optimum level of output and price that generates the maximum profit.
Short Run
An economic phase where at least one factor, like the size of a facility, remains constant and unalterable.
Regulatory Commission
A government agency or body responsible for enforcing specific laws, regulations, and standards in various sectors, including utilities, telecommunications, and financial markets.
Q18: A homopolymer is a polymer in which
Q23: The equilibrium constant for the formation of
Q34: When an acetic acid solution is titrated
Q56: Which one of the following is a
Q79: Three common weak bases are phosphate (P;
Q86: Addition of products to a chemical reaction
Q94: What is the K<sub>a</sub> for nitrous acid,
Q102: Which statement characterizes the following table? Temperature
Q113: Which one of the following is a
Q173: A sealed tube containing an equilibrium mixture