A sealed tube containing an equilibrium mixture of NO2(g) (brown) and N2O4(g) (colorless) is subjected to a variety of temperatures. For the equilibrium reaction shown, the values of the 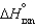 and
and 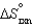 are also shown. What is observed in the tube with increasing temperature?
are also shown. What is observed in the tube with increasing temperature? 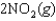
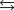
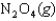 H 58.02 kJ/mol, S176.5 J/mol K
H 58.02 kJ/mol, S176.5 J/mol K
Definitions:
Interstate Commerce
The buying, selling, or moving of products, services, or money across state borders, regulated by the federal government.
Stock Options
Financial derivatives allowing the holder to buy or sell specific shares at a predetermined price within a set time frame.
Customary Type
Practices or items that are traditional or expected within a certain context or society.
Dividend
A portion of a company's earnings distributed to its shareholders, usually in the form of cash or additional stock.
Q2: Identify the weakest and strongest acids in
Q7: Low-level nuclear waste from medical facilities generally
Q7: What are the characteristics of a pH
Q23: The equilibrium constant for the formation of
Q40: What are the signs of <font face="symbol"></font>H
Q49: Identify whether or not perturbations A-D will
Q83: A scientist conducts an experiment to determine
Q122: The pH of vinegar is 2.4, and
Q149: Copper is oxidized by nitric acid. If
Q150: Identify the process that converts a neutron