Water can be separated into its elements according to the following equation by electrolysis. If 20.0 g of water is decomposed by this method, how much oxygen gas is produced? 2H2O(l)  2H2(g)
2H2(g) 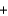 O2(g)
O2(g)
Definitions:
Opportunity Cost
The cost of foregone alternatives, representing the benefits an individual, investor, or business misses out on when choosing one alternative over another.
Interest Rate
is the cost of borrowing money or the return on investment, expressed as a percentage of the principal, over a specified period.
Q9: Which of the following graphs shows the
Q26: The integrated circuits in your cell phone
Q35: What will be the final temperature of
Q50: During winter in Siberia, a tenant in
Q70: A gallon of water has a mass
Q73: How many grams of solid magnesium chloride,
Q87: Copper(II) sulfate is a common fungicide. What
Q96: Write <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2342/.jpg" alt="Write as
Q117: Which one of the following statements is
Q136: Determine the standard enthalpy of formation for