Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 + H2O 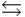 H2PO4- + H3O+
H2PO4- + H3O+ 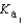 H2PO4- + H2O
H2PO4- + H2O 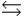 HPO42- + H3O+
HPO42- + H3O+ 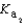 HPO42- + H2O
HPO42- + H2O 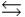 PO43- + H3O+
PO43- + H3O+ 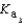 Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
Definitions:
Market Risk
The risk of losses in investments due to factors that affect the overall performance of the financial markets.
Coefficient of Variation
A statistical measure of the dispersion of data points in a data series around the mean, indicating the level of volatility.
Probability Distribution
A statistical analysis function that maps out all prospective values and their probabilities for a random variable within an established scope.
Standard Deviation
A statistical measure of the dispersion or variability of a set of data points, commonly used in finance to assess the volatility of an investment's returns.
Q4: A voltaic cell is constructed based on
Q12: Jane can accept that "<font face="symbol"></font>G<font face="symbol"></font>"
Q17: Plants convert nitrogen into ammonia. This biological
Q26: Beta emission is associated with _<br>A) conversion
Q27: In a titration of monoprotic acids and
Q42: How much work can be done using
Q49: For the reaction: Fe(H<sub>2</sub>O)<sub>6</sub><sup>3+</sup> + H<sub>2</sub>O( <img
Q50: The equilibrium constant for the formation of
Q71: Glycolic acid, which is a monoprotic acid
Q77: Smog created by the interaction of sunlight