Multiple Choice
What is the molecular formula for the compound illustrated below? 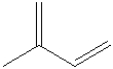
Definitions:
Related Questions
Q33: What structural characteristics must a molecule have
Q37: The concentration unit of molality is symbolized
Q41: Does the temperature of the vapors in
Q46: The following energy profiles for four different
Q51: The degree of ionization of a strong
Q51: According to band theory, when the lower
Q52: Calculate the lattice energy of sodium fluoride
Q59: Benzene is a liquid under standard conditions.
Q60: Which of the following relationships are sufficient
Q64: Consider the 1,2-dichloro derivative of ethylene in