Consider the reversible reaction at equilibrium: N2(g) + O2(g) 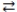 2 NO(g) . What is the effect of removing some N2(g) from the equilibrium system?
2 NO(g) . What is the effect of removing some N2(g) from the equilibrium system?
Definitions:
Lumpers
Individuals or scholars who prefer to classify information or objects into broad categories, minimizing differences within those categories.
Splitters
Individuals or researchers who emphasize distinctions and differences when categorizing objects or species, often in the context of taxonomy.
Control of Fire
The ability to create, maintain, and use fire, which is considered an important milestone in human evolution for cooking, warmth, and protection.
Human Evolutionary History
The chronology and processes through which humans evolved from primate ancestors.
Q9: Consider the following reversible reaction at equilibrium:
Q10: Rational Emotive Behavior Theory lends itself well
Q14: Energy is the capacity to do work.
Q34: Which factors increase the rate of gluconeogenesis?<br>A)high
Q40: Dilution is the addition of solute to
Q44: A saline solution used in intravenous drips
Q59: What is the molarity of a solution
Q70: How many molecules of CO<sub>2</sub> are produced
Q75: The pressure of a gas is proportional
Q77: Six of the steps of gluconeogenesis use