Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 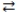 COCl2(g). If chlorine gas is added to the reaction vessel, the concentration of carbon monoxide will decrease.
COCl2(g). If chlorine gas is added to the reaction vessel, the concentration of carbon monoxide will decrease.
Definitions:
Osteoporosis
A condition characterized by weakened bones, increasing the risk of fractures.
Socioeconomic Status
A measure that combines income level, education, and occupational prestige to categorize individuals or groups.
Life Expectancy
The average number of years an individual is expected to live, based on statistical averages, taking into account factors like current age, gender, and health conditions.
Chronic Illnesses
Long-standing, persistent health conditions that typically require ongoing medical attention or limit activities of daily living.
Q7: The (II)in the name of the ionic
Q9: Consider the following reversible reaction at equilibrium:
Q17: What is the molarity of a solution
Q25: Intervening between two members who are talking
Q36: What is the molecular shape around the
Q41: The solubility of helium gas in water
Q43: The chemical formulas of ionic compounds are
Q53: The formula for the ammonium ion is
Q54: A student runs the reaction: LiOH
Q89: Oxaloacetate is the starting material in the