A certain chemical reaction sets up an equilibrium as follows
3A2(aq) + 2B3(aq) 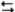 6AB(aq)
6AB(aq)
After being started with unknown amounts of A2 and B3. Once the system reaches equilibrium the concentrations of A2, B3, and AB are precisely determined and the temperature recorded. What other quantities can be determined? Q 0 is the reaction quotient at the initial conditions.
Definitions:
Frederick Taylor
Frederick Winslow Taylor was an American mechanical engineer who sought to improve industrial efficiency and is known as the father of scientific management.
Max Hawthorne
A figure not widely recognized in academic or scientific fields, potentially related to specific industries or entertainment, without a general definition available.
Chester Barnard
A business executive and author, known for his contributions to management theory, particularly in the area of organizational and executive functions.
Organizational Behavior
The study of how individuals and groups interact within organizations and the impact of these behaviors on performance and structure.
Q6: Solubility Products (K<sub>sp</sub>)<br>BaSO<sub>4</sub><br>1)5 × 10<sup>-9</sup><br>CoS<br>5)0 × 10<sup>-22</sup><br>PbSO<sub>4</sub><br>1)3
Q29: At room temperature cyclohexane exists almost exclusively
Q38: Given the reaction A(g) + B(g) <img
Q56: How many unpaired electrons does cobalt have
Q64: Given the following information, determine the standard
Q75: Calculate ΔS° at 25°C for the following
Q117: _, the study of the interactions of
Q120: Calculate the lattice energy for LiF(s) given
Q123: 150.0 mL of 1.00 M NaOH<br>A) 6.00<br>B)
Q141: Calculate ΔS° for the vaporization of Br<sub>2</sub>(l)