Based on the following electrochemical cell,which statement is true? 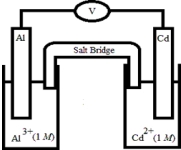 Half-Reaction E° (V) Al3+(aq) + 3e- → Al(s) -1.66
Half-Reaction E° (V) Al3+(aq) + 3e- → Al(s) -1.66
Cd2+(aq) + 2e- → Cd(s) -0.40
Definitions:
Sterilizing (Autoclave)
The process of using an autoclave to eliminate all forms of microbial life, including spores, by applying steam under pressure.
Celsius
A scale for measuring temperature where water freezes at 0 degrees and boils at 100 degrees under standard conditions.
Classification
The process of grouping objects or information based on shared qualities or characteristics.
Fevers
A rise in body temperature above the normal range, often a symptom of infection or illness.
Q12: Which isotope,when bombarded with nitrogen-15,yields four neutrons
Q20: In the complex ion [Fe(CN)<sub>6</sub>]<sup>4-</sup>,what is the
Q22: Which is the thermodynamic condition for a
Q31: What is the layer of the atmosphere
Q37: Which is a Lewis acid but not
Q54: The rate constant of the reaction Cl
Q92: Which is the correct cell diagram for
Q114: For the endothermic reaction A<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6485/.jpg"
Q115: The endpoint is used to estimate the
Q126: Below is a representation of the sparingly