The equilibrium constant for the reaction below is 2.5*109 at 25 C.
2 HCl (g) 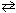 H₂ (g) + Cl₂ (g) K eq (25 C) = 3.2*10- 34
H₂ (g) + Cl₂ (g) K eq (25 C) = 3.2*10- 34
What can be stated about the relative equilibrium distribution for this mixture?
Definitions:
Performing Stage
The phase in team development where members are effectively and efficiently working together towards their common goals.
Dysfunctional Decision-making
A process where decision-making within an organization is hindered or impaired, often leading to undesirable outcomes.
Immature Group
A group in its early developmental stage, characterized by dependence, lack of norms, and undefined roles among its members.
Flexible Operating Procedures
Operational methods that allow adjustments or variations to accommodate changing conditions or needs.
Q2: Consider the Haber process for preparing ammonia
Q30: What type of crystal forces hold the
Q53: What is the molar solubility of Ag<sub>2</sub>SO<sub>4</sub>
Q66: In the titration of 0.100 M HCl
Q88: At a minimum, what experimental data is
Q92: Refer to Exhibit 14-3. What equilibrium expression
Q120: Arrange the following solutions in order of
Q131: The solubility of gases in a liquid
Q204: What is the solubility of PbI<sub>2</sub> (
Q207: At high temperature, the following gas phase