Some KCl is dissolved in water at 25°C, where it completely dissociates.The vapor pressure of pure water at 25°C is 28.3 mmHg.On the graph below, sketch the vapor pressure above the salt solution as a function of the mole fraction of H2O, assuming that Raoult's law is obeyed.Explain how you arrived at your graph. 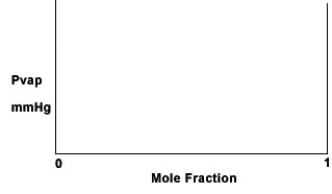
Definitions:
Q17: Consider the equilibrium equation C(s)+ H<sub>2</sub>O(g)
Q17: Identify the conjugate acid of CO<sub>3</sub><sup>2-</sup><br>A)H<sub>2</sub>CO<sub>3</sub><br>B)HCO<sub>3</sub><sup>-</sup><br>C)H<sub>2</sub>O<br>D)H<sub>3</sub>O<sup>+</sup><br>E)CO<sub>2</sub>
Q30: The N - N - H bond
Q40: Assuming the octet rule is obeyed, how
Q40: For the reaction BrO<sub>3</sub><sup>- </sup>+ 5Br<sup>-</sup>+
Q67: The number of atoms in a face-centered
Q76: The equilibrium constants for the chemical
Q95: The half life for a first order
Q95: A quantity of liquid methanol, CH<sub>3</sub>OH, is
Q103: Which one of the following substances should