The brown gas NO2 and the colorless gas N2O4 exist in equilibrium, 2NO2 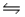 N2O4.In an experiment, 0.625 mole of N2O4 was introduced into a 5.00 L vessel and was allowed to decompose until equilibrium was reached.The concentration of N2O4 at equilibrium was 0.0750 M.Calculate Kc for the reaction.
N2O4.In an experiment, 0.625 mole of N2O4 was introduced into a 5.00 L vessel and was allowed to decompose until equilibrium was reached.The concentration of N2O4 at equilibrium was 0.0750 M.Calculate Kc for the reaction.
Definitions:
Language Reaction Hypothesis
Suggests that the language one speaks influences their cognitive processes and reaction to stimuli.
Dynamic Hypothesis
A theory proposing that complex systems or patterns of behavior are characterized by continuous change, adaptation, or progression.
Morphemes
The smallest grammatical units in a language that carry meaning, such as prefixes, roots, and suffixes.
Phonemes
The minimal sound element in a language that differentiates one word from another.
Q1: Arrange the following substances in order of
Q7: The pH of a 0.6 M solution
Q8: The equilibrium constant for the reaction Ni(s)+
Q19: Two moles of PCl<sub>5</sub> are placed in
Q44: Which one of the following would be
Q56: For the reaction SbCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q69: The molar enthalpy of vaporization of hexane
Q96: Sodium carbonate can be made by
Q110: Based on the phase diagram shown below,
Q114: Consider the reaction CO(g)+ 2H<sub>2</sub>(g) <img