Multiple Choice
Consider the weak bases below and their Kb values: 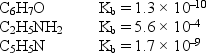 Arrange the conjugate acids of these weak bases in order of increasing acid strength.
Arrange the conjugate acids of these weak bases in order of increasing acid strength.
Discuss the impact of gender and societal roles on children's preferences and behaviors.
Describe key milestones in emotional and social development, including emotional regulation, stranger anxiety, and attachment processes.
Understand the concept of excise taxes and their impact on market prices.
Analyze how the imposition of taxes affects producer and consumer behavior.
Definitions:
Related Questions
Q15: The half-reaction occurring at the cathode
Q15: Is the reaction SiO<sub>2</sub>(s)+ Pb(s) <span
Q18: Consider the following standard reduction potentials in
Q30: What is the mole fraction of sodium
Q67: Complete and balance the following redox
Q74: Equilibrium constants are known for the following
Q75: For the reaction at equilibrium: 3Fe(s)+ 4H<sub>2</sub>O(g)
Q90: Write a net ionic equation for the
Q122: Write the formula for the conjugate base
Q163: Which one of these equations represents