Predict the direction in which the equilibrium will lie for the reaction H2SO3(aq) + HCO3- (aq) 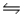
HSO3-(aq) + H2CO3(aq) .
Ka1(H2SO3) = 1 × 10-2; Ka1(H2CO3) = 4.2 × 10-7
Definitions:
Negatively Skewed
A distribution shape where the tail is longer on the left side of the distribution's peak, indicating that the bulk of the values are towards the right.
Histogram
A type of bar chart that illustrates the distribution of a continuous dataset by dividing it into intervals or bins and showing the frequency or number of data points in each bin.
Coefficient Of Variation
A statistical measure that compares the standard deviation to the mean of a dataset, expressed as a percentage, used to assess relative variability.
Variability
A measure of the dispersion or spread of a set of data points within a dataset, indicating how much the data points differ from the average value.
Q3: Write the formula of the weakest reducing
Q24: For the reaction at equilibrium 2SO<sub>3</sub>
Q33: The equilibrium constant for the reaction
Q85: Consider this reaction at equilibrium at a
Q85: At 700 K, the equilibrium constant
Q87: Will a precipitate form (yes or no)when
Q91: Write an equation showing the net reaction
Q99: Given that the heat of vaporization of
Q111: Suppose the atoms in a two-dimensional crystal
Q130: The measured voltage of a cell