Calculate the equilibrium constant for the decomposition of water 2H2O(l) 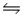
2H2(g) + O2(g)
At 25°C, given that G°f (H2O(l) ) = -237.2 kJ/mol.
Definitions:
Ace of Spades
A playing card with a single spade symbol and an ace rank, often considered to be the highest card in card games.
Ace of Hearts
One of the 52 playing cards in a standard deck, recognized by a single heart symbol and an 'A' indicating its rank.
King of Spades
A playing card in the spade suit, traditionally depicted with a king holding a sword.
Independent Events
Events wherein the happening of one does not alter the chance of occurrence of the others.
Q9: Calcium metal is produced by electrolysis of<br>A)CaSO<sub>4</sub><br>B)CaF<sub>2</sub><br>C)CaCO<sub>3</sub><br>D)Ca(OH)<sub>2</sub><br>E)CaCl<sub>2</sub>
Q68: Petroleum is a fossil fuel containing many
Q76: The equilibrium constants for the chemical
Q85: At 700 K, the equilibrium constant
Q89: What is the nuclear binding energy per
Q109: Predict the direction in which the equilibrium
Q115: Consider the following decay series: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q116: The K<sub>sp</sub> for silver(I)phosphate is 1.8 ×
Q120: Nitrosyl chloride (NOCl)decomposes at elevated temperatures
Q136: The pH of a 0.14 M solution