Carbon tetrachloride reacts at high temperatures with oxygen to produce two toxic gases, phosgene and chlorine. CCl4(g) + 1/2O2(g) 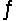
COCl2(g) + Cl2(g) , Kc = 4.4 * 109 at 1,000 K
Calculate Kc for the reaction 2CCl4(g) + O2(g) 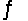
2COCl2(g) + 2Cl2(g) .
Definitions:
Supreme Court
The highest federal court in the United States, consisting of nine justices and having jurisdiction over all other courts in the nation.
Regulated
Controlled or governed by rules, statutes, or laws set forth by a government or regulatory authority to ensure fairness, safety, or compliance.
Bill of Rights
The initial ten amendments to the U.S. Constitution, providing crucial liberties and protections for citizens of the United States.
Open Debate
A discussion or argumentation that is unrestricted and accessible to all interested parties, allowing for the free exchange of ideas.
Q42: The density of a 20.3 M CH<sub>3</sub>OH
Q64: Use the table of data shown below
Q77: For the following reaction at equilibrium
Q82: The activation energy for a certain reaction
Q83: Methyl red is a common acid-base indicator.
Q88: What is the rate law that corresponds
Q89: What is the approximate Na<sup>+</sup> ion concentration
Q89: At 1500°C the equilibrium constant for
Q99: The isomerization of cyclopropane to form propene
Q134: Indicate all the types of intermolecular forces