For the nitrogen fixation reaction 3H2(g) + N2(g) 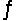 2NH3(g) , Kc = 6.0 * 10-2 at 500°C. If 0.250 M H2 and 0.050 M NH3 are present at equilibrium, what is the equilibrium concentration of N2?
2NH3(g) , Kc = 6.0 * 10-2 at 500°C. If 0.250 M H2 and 0.050 M NH3 are present at equilibrium, what is the equilibrium concentration of N2?
Definitions:
Dewpoint
The temperature at which air must be cooled, at constant pressure and water vapor content, for it to reach saturation and produce dew.
Linear Regression
A statistical method used to model the relationship between a dependent variable and one or more independent variables by fitting a linear equation to observed data.
Least-square Regression
Rephrasing of KT-14; Refers to the method used to find the line that minimizes the sum of the squares of the differences between observed and predicted values.
Vector-borne Disease
Infectious diseases transmitted by vectors, such as mosquitoes, ticks, and fleas, that spread pathogens between humans or from animals to humans.
Q7: For the reaction at equilibrium 2SO<sub>3</sub>
Q8: Predict the direction in which the equilibrium
Q13: The first-order decomposition of SO<sub>2</sub>Cl<sub>2</sub> to sulfur
Q30: Potassium crystallizes in a body-centered cubic lattice.How
Q50: What volume of ethanol (density = 0.7893
Q61: In the reaction CaO(s)+ SO<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q74: Using the thermodynamic data provided below, determine
Q95: Consider a 0.90 M Al(NO<sub>3</sub>)<sub>3</sub> solution. This
Q106: The atomic planes in a graphite crystal
Q124: The equilibrium constant for the reaction C<sub>6</sub>H<sub>5</sub>COOH(aq)+