Multiple Choice
Predict the direction in which the equilibrium will lie for the reaction H3PO4(aq) + HSO4-(aq) 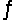 H2PO4-(aq) + H2SO4(aq) .
H2PO4-(aq) + H2SO4(aq) .
Ka1(H3PO4) = 7.5 * 10-3; Ka(H2SO4) = very large
Comprehend the debates surrounding the relationship between Neandertals, Denisovans, and anatomically modern humans.
Recognize the technological advancements and cultural developments during the Upper Paleolithic era.
Identify the factors influencing genetic diversity and species differentiation.
Explain the significance of heritable traits in evolutionary change.
Definitions:
Related Questions
Q5: Equilibrium constants are known for the following
Q33: Nitrous oxide (N<sub>2</sub>O)decomposes at 600°C according to
Q40: A solution is 40.0% by volume benzene
Q59: For the following reaction at equilibrium
Q60: How does the entropy change when a
Q80: The thermal decomposition of acetaldehyde, CH<sub>3</sub>CHO <font
Q92: The data below refer to the following
Q107: Calculate the amount of heat needed
Q115: Calculate the minimum voltage required for
Q161: Predict the direction in which the equilibrium