Predict the direction in which the equilibrium will lie for the reaction C6H5COO- + HF 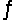 C6H5COOH + F- .
C6H5COOH + F- .
Ka(C6H5COOH) = 6.5 *10-5; Ka(HF) = 7.1 *10-4
Definitions:
Locations
Specific places or positions within a given space or area.
Zygote
The initial cell formed when two gamete cells are joined by means of sexual reproduction, containing genetic information from both parents.
Gamete
A mature sexual reproductive cell, such as a sperm or egg, that unites with another cell to form a new organism.
Random Chromosome Alignment
The process during meiosis where chromosomes arrange themselves randomly at the equatorial plate, which contributes to genetic variation.
Q12: The equilibrium constant for the reaction C<sub>7</sub>H<sub>15</sub>COOH(aq)+
Q19: What concentration of Ni<sup>2+</sup> ion remains in
Q25: For the overall chemical reaction shown below,
Q33: What is the mole fraction of sodium
Q42: On analysis, an equilibrium mixture for the
Q47: Predict the sign of <span
Q59: At 700 K, the rate constant for
Q95: For the reaction SbCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q106: The rate constant of a first-order reaction,
Q159: Calculate the pH of a 0.021 M