Consider the reaction CO(g)+ 2H2(g) 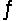 CH3OH(l)at 25°C.
CH3OH(l)at 25°C.
G°f (CO)= -137.3 kJ/mol
G°f (CH3OH)= -166.3 kJ/mol
H°f (CO)= -110.5 kJ/mol
H°f (CH3OH)= -238.7 kJ/mol
S°(CO)= 197.9 J/K·mol
S°(CH3OH)= 126.8 J/K·mol
Calculate value of the equilibrium constant (Kp)for this reaction at 25°C.
Definitions:
Psychotic Symptoms
Experiences that indicate a loss of contact with reality, such as hallucinations or delusions.
Schizophrenia
A psychiatric disorder characterized by distortions in thinking, perception, emotions, language, sense of self, and behavior.
Medication
Substances used to diagnose, treat, or prevent illness and disease, aiding in improving health or managing symptoms.
Posthospitalization Services
Care and assistance provided to patients after they have been discharged from the hospital, aimed at ensuring recovery and preventing readmission.
Q6: Consider the following equilibrium,<br>4NH<sub>3</sub>(g)+ 3O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q17: Complete and balance the following redox
Q27: What mass of ammonium chloride must be
Q32: Sulfur-35 decays by beta emission. The decay
Q47: Which one of these hydrocarbon chains would
Q53: How many unpaired electrons are there in
Q66: Consider the following standard reduction potentials in
Q71: Calcium carbonate decomposes at high temperatures to
Q78: What mass of ammonium nitrate must be
Q129: Which of these acids is the strongest?<br>A)H<sub>2</sub>SO<sub>3</sub><br>B)H<sub>2</sub>SeO<sub>3</sub><br>C)H<sub>2</sub>TeO<sub>3</sub>