Given: 2SO2(g) + O2(g) 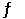 2SO3(g) At equilibrium at a certain temperature,the concentrations of SO3(g) ,SO2(g) ,and O2(g) are 0.24 M,0.82 M,and 0.33 M,respectively.Calculate the value of Kc for this reaction.
2SO3(g) At equilibrium at a certain temperature,the concentrations of SO3(g) ,SO2(g) ,and O2(g) are 0.24 M,0.82 M,and 0.33 M,respectively.Calculate the value of Kc for this reaction.
Definitions:
Consideration
The value, typically expressed in monetary terms, given in exchange for goods, services, or for entering into a contract.
Control
The power to direct the financial and operating policies of an entity with a view to gaining economic benefits from its activities.
Unconditional Contract
An agreement whose execution does not depend on fulfilling any conditions; it is binding upon the parties as soon as it is signed.
Physical Substance
The tangible, material aspect of an asset, referring to its physical existence and properties.
Q3: Why is the vapor pressure of cis-dibromoethene
Q6: The equilibrium constant expression for the reaction<br>CuSO<sub>4</sub>(s)
Q12: Consider the reaction 2N<sub>2</sub>O(g)<font face="symbol"></font> 2N<sub>2</sub>(g)+ O<sub>2</sub>(g)<br>Rate
Q20: HBrO(aq),in a 0.25 M solution,has a pK<sub>a</sub>
Q25: The reduction of M<sup>3+</sup> by Cr<sup>2+</sup> was
Q27: The standard enthalpy of formation of gaseous
Q33: What is the concentration of acetate ion
Q40: To what is the boron nitride structure
Q54: Calculate the standard enthalpy of combustion of
Q91: The osmotic pressure of 1.00 g of