A flask contains the following chemical system at equilibrium.
CuCO3(s) 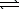 Cu2+(aq) + 2 CO32-(aq)
Cu2+(aq) + 2 CO32-(aq)
Addition of which of the following substances will increase the solubility of CuCO3(s) in water?
1) aqueous hydrochloric acid
2) aqueous sodium carbonate
3) solid copper(II) carbonate
Definitions:
Rational Exponents
Fractional exponents, with the numerator indicating the exponentiation level and the denominator designating the root level of the primary number.
Simplify
The process of making an expression easier to understand by reducing it to its simplest form.
Expression
A combination of symbols and numbers to represent a mathematical operation or quantity.
Radical Expression
An expression that contains a square root, cube root, or another root.
Q12: A solution containing 10.mmol of CO<sub>3</sub><sup>2-</sup> and
Q39: At a given temperature,0.0664 mol N<sub>2</sub>O<sub>4</sub>(g)is
Q42: The K<sub>a</sub> for the monoprotic acid
Q46: At 25 <sup> <span class="ql-formula" data-value="\circ"><span
Q52: The lattice energy of NaBr(s)is -751
Q53: If electric current is passed through a
Q63: Calculate the cell potential,at 25 <sup>
Q69: A molecule will <span class="ql-formula"
Q75: Draw the reaction coordinate diagram for an
Q80: The primary source material for the commercial